Abstract
Drug resistance is a major problem in the treatment of cancer, particularly in hematological malignancies where many patients relapse and become refractory to current standard of care chemotherapy (SOC). In Acute Myeloid Leukemia (AML) and Diffuse Large B-cell Lymphoma (DLBCL), relapse post-SOC occurs in about 10-40% and 40% of patients respectively (Shi, Das et al. 2013, Thol, Schlenk et al. 2015). This highlights the critical need for the identification of drug resistance mechanisms to help physicians make better clinical decisions and improve patient outcomes. Given the emerging role of the tumor microenvironment in mediating drug resistance, we employed a targeted, quantitative metabolomics approach, utilizing Mass Spectrometry and the AbsoluteIDQ p180 Kit from Biocrates Life Sciences to identify predictive and prognostic biomarkers. We hypothesize one such mechanism of resistance involves catabolism of the essential amino acid Tryptophan (Trp). The catabolism of tryptophan is a central pathway maintaining an immunosuppressive microenvironment in many cancer types by regulating both innate and adaptive immunity. Trp can be catabolized by tryptophan hydroxylase into serotonin. Alternatively, it can be catabolized by Indoleamine-2,3-dioxygenase (IDO) and Tryptophan-2,3-dioxygenase (TDO) into kynurenine (kyn). IDO/TDO driven production of kynurenine promotes the development, stabilization, and activation of Tregs, while suppressing effector T cells (pro-inflammatory Th1 and Th17 pathways) all of which contribute to immune system impairment in cancer bearing individuals.
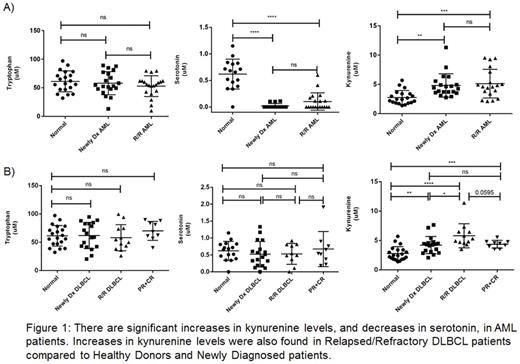
A pilot study aimed at evaluating changes in the tryptophan pathway in human serum samples from commercially available AML and DLBCL patients who had undergone standard of care treatments was performed. This was accomplished through mass spectrometry analysis of tryptophan, kynurenine, and serotonin, along with 185 additional metabolites, using the Absolute IDQ p180 kit by Biocrates. Analysis was performed using MetaboAnalyst 3.0 and Graphpad prism software. As seen in Figure 1A, we found significant increases in kynurenine in the serum from the commercially available Newly Diagnosed (Newly Dx) (n=20, p<0.001) and Relapsed/Refractory (R/R) AML patients (n=19, p<0.001) compared to normal donors (n=20). We also observed additional defects in the tryptophan pathway with significantly lower serotonin production (p<0.0001) in the malignant population. Similarly, in Figure 1B, we showed that Newly Dx DLBCL patients had significantly elevated levels of kynurenine (n=18, p<0.01) compared to normal donors (n=20), and the R/R population was elevated even further (n=12, p<0.001), with a trend towards decreased kynurenine levels in remission patients (n=9, p=0.0595). Interestingly, the ratio of Kyn/Trp (indicative of IDO activity) is significantly decreased in the remission population (p<0.05) when compared to the R/R DLBCL population, suggesting this might serve as a prognostic biomarker. Unlike in AML, no significant changes in serotonin were observed. Given these results, a retrospective study is underway to establish which will serve as the best biomarkers (predictive and prognostic) for patients treated with Amgen therapies.
Finger: Amgen, Inc.: Employment, Equity Ownership. Wong: Amgen, Inc.: Employment, Equity Ownership. Gray: Amgen, Inc.: Employment, Equity Ownership. Rock: Amgen, Inc.: Employment, Equity Ownership. Loberg: Amgen Inc.: Employment, Equity Ownership. Fitzpatrick: Amgen Inc.: Employment, Equity Ownership. Smith: Amgen, Inc.: Employment, Equity Ownership. Wang: Amgen, Inc.: Employment, Equity Ownership. Dos Santos: Amgen Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal